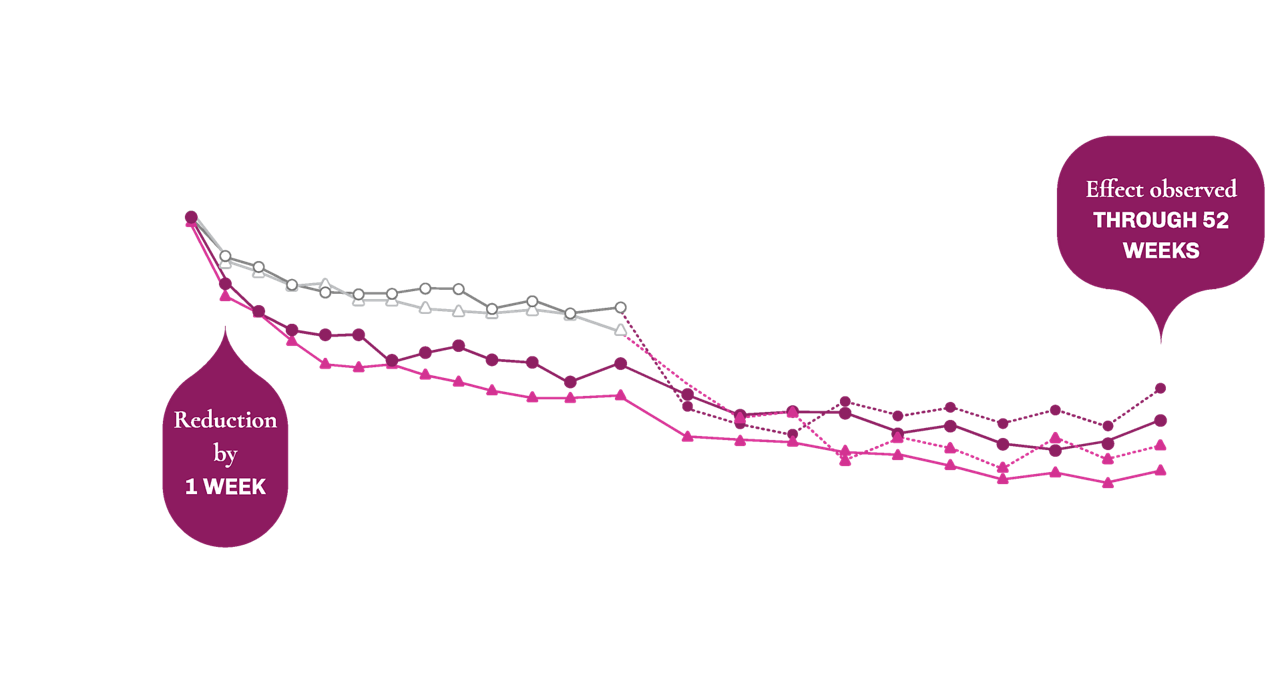
Select inclusion criteria1,2:
- Born female, aged ≥40 to ≤65 years, with moderate to severe VMS due to menopause (minimum average of 7 moderate to severe VMS episodes per day or 50 to 60 per week) and confirmed menopausal
Menopause was defined as: spontaneous amenorrhea for ≥12 consecutive months, spontaneous amenorrhea for ≥6 months with biochemical criteria of menopause (follicle-stimulating hormone >40 IU/L), or bilateral oophorectomy ≥6 weeks before the screening visit (with or without hysterectomy)
- Body mass index ≥18 kg/m2 and ≤38 kg/m2
Select exclusion criteria1-4:
- History of undiagnosed uterine bleeding within the last 6 months
- Unacceptable result from the transvaginal ultrasound assessment at screening
- Endometrial biopsy confirming presence of disordered proliferative endometrium, endometrial hyperplasia, endometrial cancer, or other clinically significant findings at screening
Participants in the study5:
- Self-identified as Caucasian (81%), African American (17%), Asian (1%), Hispanic/Latina ethnicity (24%). The mean age was 54 years
- Included menopausal women with prior hormone therapy use (19.9%), with oophorectomy (21.6%), or with hysterectomy (32.1%)
REFERENCES: 1. Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet 2023;401(10382):1091-102. 2. Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate to severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab (Epub) 02-03-2023. 3. ClinicalTrials.gov. A study to find out if fezolinetant helps reduce moderate to severe hot flashes in women going through menopause (Skylight 1) (08-12-2022). https://clinicaltrials.gov/ct2/show/NCT04003155. Accessed 08-15-2022. 4. ClinicalTrials.gov. A study to find out if fezolinetant helps reduce moderate to severe hot flashes in women going through menopause - 2 (Skylight 2) (07-18-2022). https://clinicaltrials.gov/ct2/show/NCT04003142. Accessed 08-15-2022. 5. VEOZAH [package insert]. Northbrook, IL: Astellas Pharma US, Inc.